Le premier essai (FM 57) était une étude multicentrique, en double aveugle, d’une durée de 12 semaines, ayant inclus 250 hommes utilisant Eroxon®. Avant d’utiliser le traitement, les participants ont été suivis pendant 4 semaines afin de déterminer l’étendue de leur dysfonctionnement érectile. cette dernière a été mesurée à l’aide de trois mesures autorisées au niveau international : IIEF-EF, SEP2 et SEP3.
L’efficacité s’est améliorée après la première dose et les hommes ont obtenu une érection dans les 10 minutes dans 60 % des applications. Il y a eu 3 792 tentatives de rapports sexuels et deux tiers (63 %) des hommes ont atteint ou dépassé la différence minimale cliniquement importante (DMCI), qui est le seuil de réponse significative.
À 12 semaines, les utilisateurs d’Eroxon® Stimgel ont présenté une amélioration significative sur toutes les mesures initiales, la réponse augmentant linéairement avec la sévérité de leur dysfonctionnement érectile. sur 5 hommes présentant la forme la plus sévère de dysfonctionnement érectile, 4 (80 %) ont atteint et, dans certains cas dépassé, la dmci. parmi les hommes souffrant de dysfonctionnement érectile léger et modéré, 61 % et 59 %, respectivement, ont atteint ou, dans certains cas dépassé, la DMCI.
Les effets indésirables déclarés étaient minimes.
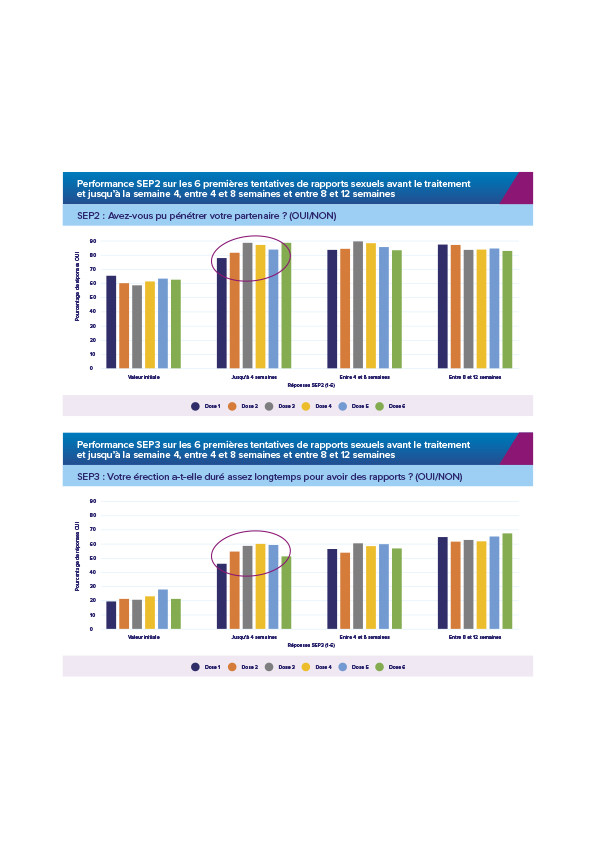
PERFORMANCE SEP 2 ET SEP 3 DANS L’ÉTUDE FM57 SUR LES DOSES 1-6
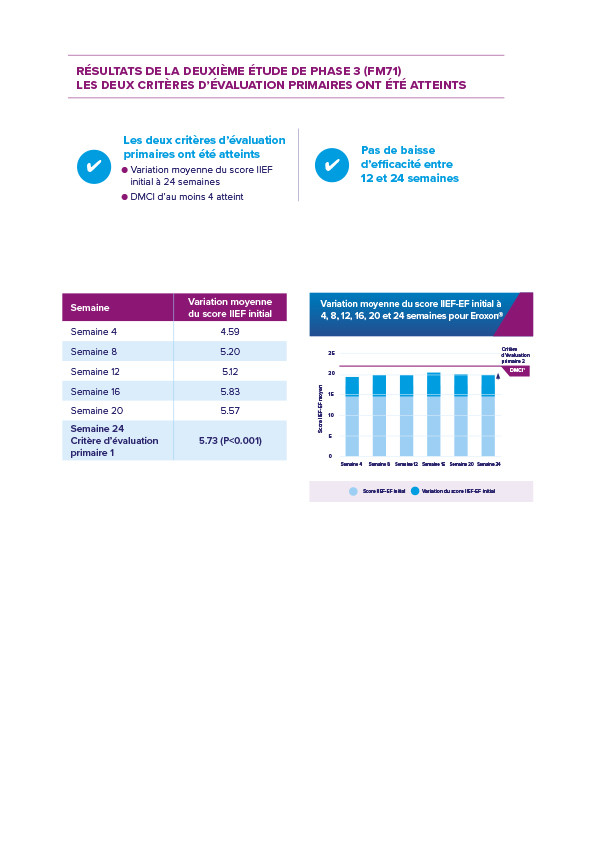
Le deuxième essai de phase 3 (FM71) était une étude de 24 semaines conçue en vertu du retour d’information de la FDA afin d’exclure tout effet placebo. L’étude comportait une comparaison directe avec un médicament per os.
Les résultats ont été présentés lors du congrès 2023 de la European Society for Sexual Medicine (ESSM).
L’étude FM71 a montré qu’Eroxon® Stimgel atteignait la DMCI chez 61 % des hommes souffrant de dysfonctionnement érectile à 24 semaines (1 551 tentatives de rapports sexuels) ; 55 % des hommes atteints de dysfonctionnement érectile léger, 51 % des hommes atteints de dysfonctionnement érectile modéré et 87 % des hommes atteints de dysfonctionnement érectile sévère atteignant cet objectif clinique.
L’efficacité a augmenté au fil de l’étude.
Sur la base de ces preuves cliniques, Eroxon® Stimgel a été approuvé dans l’UE et au Royaume-Uni comme étant le premier traitement topique du dysfonctionnement érectile en vente libre à l’efficacité cliniquement prouvée et peut être acheté sans ordonnance.



